Case Study: Contract Manufacturing Diagnostic
August 2, 2024
SMC’s range of autoinjector platform technologies combine patient-friendly designs with the ability to deliver a wide range of drug formulations for both subcutaneous and intramuscular injections. Customizable for your formulation, the family (trio) has been designed with a range of 0.5-5ml and viscosity up to <1000cp to fit your needs.

The ArQ® subcutaneous platform features a simple, two step user interface with audible clicks providing feedback at the start and end of drug delivery. Capable of meeting a range of viscosities and volumes, via various needle gauge options, ArQ is compatible with your existing fill-finish processes.
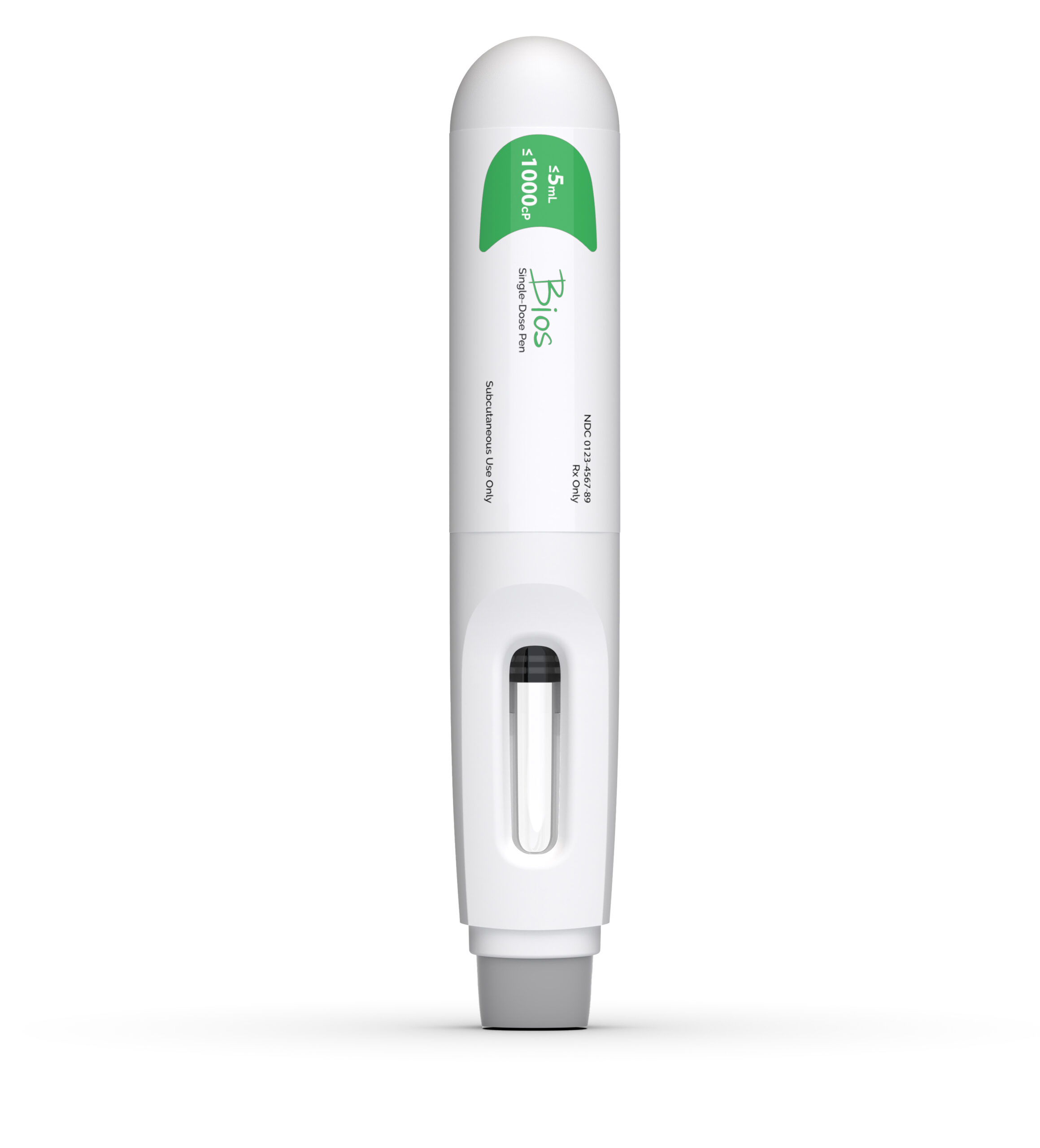
The Bios platform delivers on the increasingly requirement for large volume, ultra-high viscosity formulations. Utilizing ISO-standard plunger stoppers and staked-needle syringes (glass or plastic), Bios fulfills the complex injection needs of a wide range of challenging formulations.

The Vita platform has been designed to deliver large volumes and includes resuspension technology, enabled by a dry-needle system, suitable for long-acting formulations. Vita is compatible with ISO-standard plunger stoppers.
Our team delivers the full range of CDMO services for a variety of delivery mechanisms including injectables, inhalation, OBDS, transdermal and custom devices.
Yes, SMC is a global organization with 11 locations – United Kingdom, India, Costa Rica and 9 located in the United States (California, Ohio, Wisconsin (4), Massachusetts, North Carolina).
specialties. For example: SMC Somerset specializes in silicone manufacturing. North Carolina serves as our dedicated fill-finish location. Our facilities are strategically located to provide convenient access and support to our customers. To determine which facility is best suited for your project, we recommend reaching out to us for personalized guidance. Contact us for more information.
All of SMC manufacturing fall in either Medical Device, Diagnostics or the Pharmaceutical Markets. We do not manufacture anything in other markets.
At SMC, we stand out among other CDMOs (Contract Development and Manufacturing Organizations) because of our unique approach and capabilities. Not only are we a privately held company with an actively engaged management team, but we also specialize exclusively in medical manufacturing, serving the medical device, diagnostic, and pharmaceutical markets.
Our end-to-end manufacturing solutions cover everything from design to production, including fill-finish capabilities. With subject matter experts (SMEs) available every step of the way, we ensure a seamless process tailored to your needs.
What truly sets SMC apart is our proprietary auto-injector technologies, which we offer exclusively to our customers.
At SMC, the sooner we can get involved, the better! However, we’re happy to assist at any stage of your project. Whether you’re starting with a simple concept—like a napkin sketch—or transferring a fully developed program through a lift-and-shift process, we’re here to help.
It’s always best to engage your Contract Manufacturer (CM) as early as possible during your program’s planning phase. Early collaboration allows us to partner with you effectively and thoroughly review the manufacturing aspects of your project for optimal success.
At SMC, the collaborative supply chain teams, led by our Chief Supply Chain Officer, employ best-in-class techniques to ensure customer success on a global scale. The SMC-approved supplier list includes multiple alternatives for all major commodities, providing diverse sourcing options to mitigate risk. We collaborate closely with internal stakeholders, including finance, engineering, operations, and legal teams, to ensure alignment and support for procurement strategies tailored to address customer concerns and needs. By building and nurturing strong relationships with key suppliers and partners, we leverage strategic advantages that foster innovation, enhance pricing power, and provide flexibility in production and transportation.
Key performance indicators (KPIs) are utilized to measure the effectiveness of procurement strategies and operations, driving continuous improvement in supply base processes, systems, and practices. Our approach anticipates and mitigates risks by accounting for economic, geopolitical, and environmental events that may impact procurement and supply chain operations. Additionally, we achieve buying power through a combination of concentrated spending with key suppliers, independent pricing and minimum order quantity (MOQ) negotiations, and the use of purchasing data and forecast extrapolations.
Our success in managing potential supply risk is rooted in our focused approach across several key areas. We maintain a strong core of senior technical and commercial leaders within SMC, ensuring that experienced leadership drives risk management efforts. Identifying supply risk areas and implementing mitigation strategies is a core responsibility of our senior management and their teams. To stay ahead of our customers’ capacity needs, we pre-invest in infrastructure, ensuring preparedness and adaptability. Consistent technology investments enable us to mitigate risks related to both obsolescence and security.
Additionally, we provide safety stock of both raw materials and finished goods to support our customers’ needs. Where applicable, we employ dual sourcing for critical components to enhance supply chain resilience. Lastly, site-specific business continuity plans allow us to respond quickly and effectively to unexpected events, minimizing potential disruptions.
Yes, all SMC Facilities are ISO 13485 Certified and FDA registered. You Can find all of our certifications here.
Our contract manufacturing capabilities, focused on single-use and disposable medical devices, include innovative autoinjector platforms designed for subcutaneous or intramuscular injection. These platforms can be customized to your drug formulation.