Autoinjectors, Ready To Use, Right Now
SMC's range of autoinjector platform technologies combine patient-friendly designs with the ability to deliver a wide range of drug formulations for both subcutaneous and intramuscular injections. Customizable for your formulation, the family is available for commercial launch right now.

ArQ® subcutaneous platform
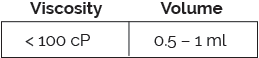
The ArQ® subcutaneous platform features a simple, two step user interface with audible clicks providing feedback at the start and end of drug delivery. Capable of meeting a range of viscosities and volumes, via various needle gauge options, ArQ is compatible with your existing fill-finish processes.

Bios subcutaneous platform
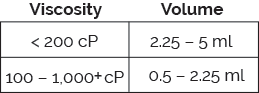
The Bios platform delivers on the increasingly requirement for large volume, ultra-high viscosity formulations. Utilizing ISO-standard plunger stoppers and staked-needle syringes (glass or plastic), Bios fulfills the complex injection needs of a wide range of challenging formulations.

Vita resuspension platform
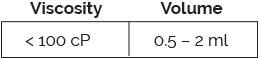
The Vita platform has been designed to deliver large volumes and includes resuspension technology, enabled by a dry-needle system, suitable for long-acting formulations. Vita is compatible with ISO-standard plunger stoppers.
Both the gauge and extended length of the needle are configurable to suit your specific application, with automatic insertion and retraction for a simple, intuitive patient experience.
How can we help you suceed?
Let's start the conversation.